Effects and examples related to throttle cooling
Effect: Positive Joule-Thomson effect (cooling of gas)
Example: Fast cooling
Example: Throttle cooling of gas
![]()
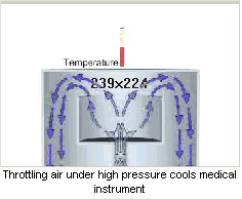
Description
The medical instrument is an assembly of two metal jackets. Inside the jackets, there is a gas expansion chamber. Air under high pressure is charged into the chamber through a narrow (0.12 mm) orifice.
Inside the chamber, the air is intensively expanded and cooled. Such an effect is called the positive (positive Joule-Thomson effect). A cool air flow quickly (in less than 15 seconds) cools the medical instrument down to 203 K (-70 0 C).
See Also
Effect: Positive Joule-Thomson effect (cooling of gas)
Advantages
Cheapness. Compressed air is much cheaper than liquid nitrogen.
Problem
To be destroyed, a malignant tissue is brought into contact with a cooled medical instrument. Usually, it is a probe-heat exchanger through which liquid nitrogen is passed.
However, the production, transportation and storage costs of liquid nitrogen make this method of cooling malignant tissue very expensive.
Solution
In order to cool medical instrument,
it is proposed to throttle (discharge through a narrow orifice) air under high pressure.
References
U.S. Patent. 5,522, 870; Ben-Zion; Jun. 4, 1996; “Fast changing heating - cooling device and method"; State of Israel, Ministry of Defense, Rafael Armaments Development Authority, Israel, Maytal Ben – Zion.
![]()
Example: Throttle cooling of gas
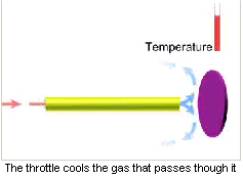
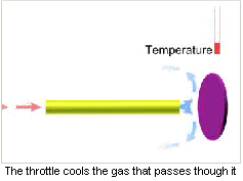
Description
The semiconducting detectors are blown over by a cooled or liquefied gas for cooling. For cooling purposes the gas is passed under pressure through a diaphragm with a small opening or through a pipe of a small diameter (throttle). In consequence, its temperature lowers. The gas cooling degree can be regulated. A coolable detector is positioned at the throttle output.
See Also
Effect: Joule-Thomson effect
Example: Fast heating
Example: Gases with negative Joule-Thomson effect
Advantages
The gas temperature at the throttle output lowers without additional means.
Problem
It is necessary to keep infrared radiation detectors at low temperatures for their good operation. They are usually cryogenic temperatures. It is difficult to store cooled or liquefied gases for a long time. Compression liquefiers are complicated in operation.
Solution
In order to cool gas,
it is proposed to pass the gas through a throttle.
References
U.S. Patent. 4,621,279; Maier, et al.; Nov. 4, 1986; “Non-evacuated, rapidly coolable housing for an opto-electronic semiconductor component”; Telefunken Electronic GmbH, Heilbroom.
![]()
Positive Joule-Thomson effect (cooling of gas)
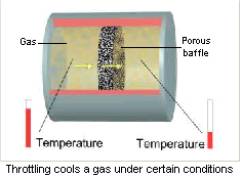
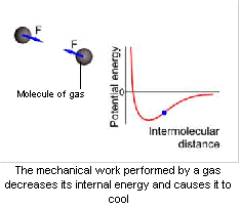
The Joule-Thomson effect is the change in the temperature of a gas during adiabatic throttling. In other words, it is the temperature change of a gas that flows slowly through a throttle under a constant difference in pressure. The throttle is a local obstacle to the gas flow, such as a porous partition in the tube).
Intermolecular forces cause gas molecules to interact. When gas molecules attract each other, the internal energy of the gas includes both the kinetic and potential energy of their interaction. If an energetically insulated gas expands, its internal energy does not change. However, the potential energy of interaction of its molecules increases, and the kinetic energy decreases. The increasing distance between the molecules causes these energy changes. As a result, the thermal motion of the molecules slows down, and the temperature of the gas decreases. Considerable changes in the pressure on the throttle may considerably change the temperature of the gas.
Real processes are more complex, because a gas is not insulated from the medium. Throttling does work on the gas. This work equals the change in its internal energy. If the gas is thermally insulated, the gas enthalpy does not change, because a porous partition suppresses all macroscopic motions of the gas molecules. The temperature of the gas changes with its internal energy.
This effect vanishes for an ideal gas (the molecules of an ideal gas are considered to be material points that do not interact with one another). For real gases, the initial conditions of throttling dictate the change in temperature.
Advantages
1. The Joule–Thomson effect is widely used in cryogenic engineering.
2. The Joule–Thomson effect is used to liquefy gases.
3. Gases with a critical temperature greater than the ambient temperature (such as chlorine and ammonia) can be compressed with by subsequent condensation in water-cooled heat exchangers.
Limitations
For the negative Joule-Thomson effect, m < 0.
DP = P1 - P2 > 0.
Materials
For helium, a pressure increase from 105 Pa to 2 ´ 107 Pa (at an initial temperature 290 K) corresponds to a 14 K rise in temperature at the throttle outlet.
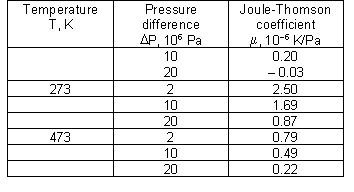
Joule-Thomson coefficient for helium at different combinations of temperature and pressure:
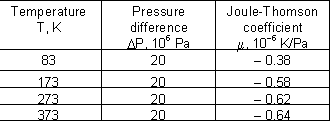
Joule-Thomson coefficient for nitrogen at different combinations of temperature and pressure
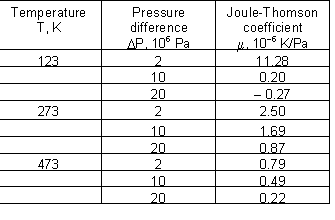
Joule-Thomson coefficient for air at different combinations of temperature and pressure:
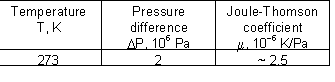
Joule-Thomson coefficient for oxygen at different combinations of temperature and pressure:
Formula
![]()
DP = P1 - P2
T2 - temperature of gas at throttle outlet, K
T1 - temperature of gas at throttle inlet, K
m - Joule–Thomson coefficient, K/Pa (Kelvin/Pascal)
DP - pressure difference under action of which throttling occurs, Pa
P1 – pressure of gas at throttle inlet, Pa
P2 – pressure of gas at throttle outlet, Pa
Conditions
1. There must be no heat exchange between the medium and the gas.
2. The throttling conditions must be selected such that the gas will be heated.
References
Encyclopedia of Physics. New York: McGraw-Hill, 1983.
Menzel, Donald H. Fundamental Formulas of Physics. New York: Prentice-Hall, 1955.
Kuchling, Horst. Physik. Leipzig: VEB Fachbuchverlag, 1980.